To find relative molecular mass from mass spectrum, identify the molecular ion peak and divide the mass by the charge. The resulting number gives the relative molecular mass.
Mass spectrometry is a useful technique used to identify unknown substances. It provides information on the molecular weight and structure of a compound. A mass spectrum graph represents the intensity of ions formed by breaking down a molecule into smaller fragments.
The molecular ion peak in the graph represents the entire molecule’s mass. By dividing the molecular ion peak’s mass by the ion’s charge, the relative molecular mass can be determined. This method helps in the identification and characterization of different compounds, including drugs, pesticides, and other organic molecules. The accuracy and reliability of mass spectrometry make it a valuable tool in various fields, including pharmaceuticals, forensics, and environmental science.
Credit: www.chemguide.co.uk
Theoretical Background
To determine the relative molecular mass from a mass spectrum, a theoretical background is necessary. The process involves analyzing the peaks in the spectrum to identify the molecular ion and its fragments, which can then be used to calculate the relative molecular mass.
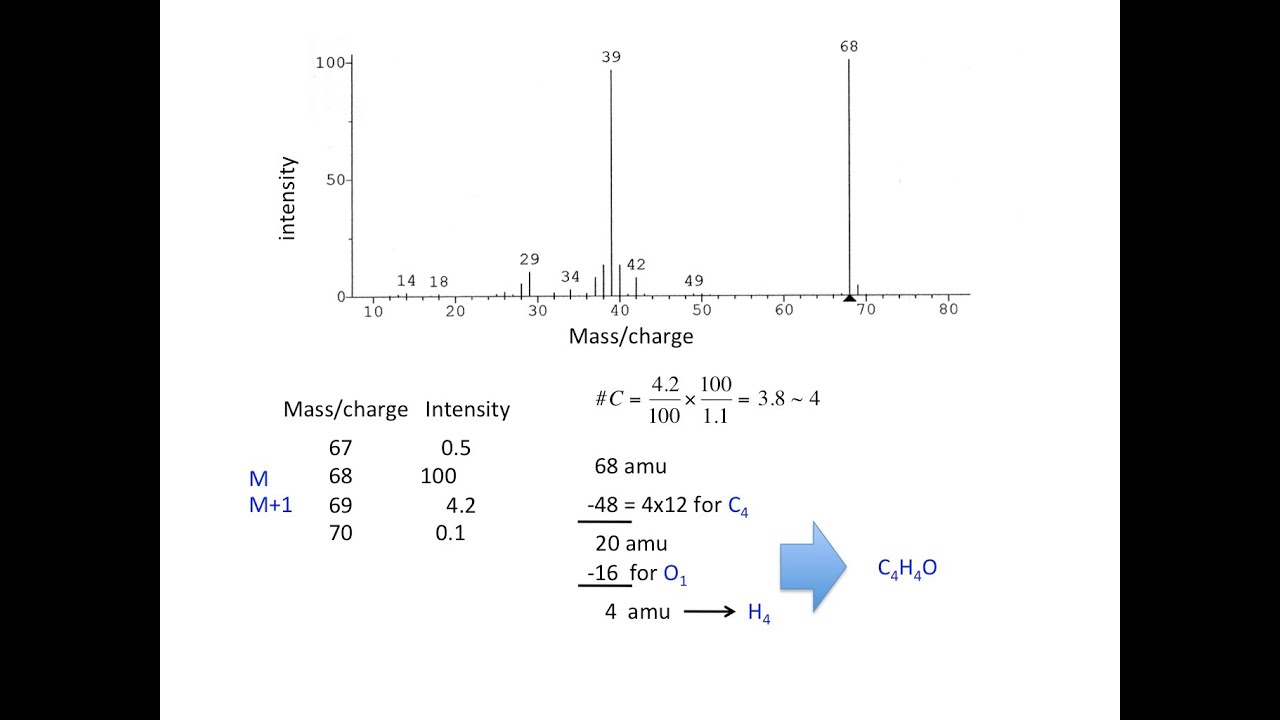
Credit: m.youtube.com
Interpreting The Spectral Data
The relative molecular mass of a compound can be determined from the mass spectrum. To do this, you need to locate the parent peak, which represents the molecular ion. This peak will have the highest m/z value and will be the peak with the greatest intensity. Once you have located the parent peak, you can identify the relative intensities of the isotopic peaks, which correspond to the presence of different isotopes of the same element in the molecule. By analyzing these peaks, you can calculate the relative molecular mass of the compound. This information is crucial in determining the identity of the compound and can be useful in a variety of applications such as drug discovery and analysis of environmental pollutants.
Calculating The Relative Molecular Mass
In mass spectrum, the isotopic peaks correspond to different isotopes of the same element. To calculate the relative molecular mass, we need to determine the isotopic peak data and the average mass. The isotopic peak with the highest intensity represents the most abundant isotope. Assume that the percentage abundance of all isotopes is known; we can calculate the average mass by multiplying the mass of each isotope by its percentage abundance, summing up the products, and dividing by 100. The average mass is then used to calculate the relative molecular mass by adding up the masses of all atoms in the molecule.
| Isotope | Mass (amu) | Percentage Abundance (%) |
|---|---|---|
| 612C | 12.0000 | 98.93 |
| 613C | 13.0034 | 1.07 |
For example, if we have two isotopes of carbon with the masses and percentage abundances as shown above, the average mass of carbon would be 12.0110 amu. To calculate the relative molecular mass of a molecule containing two carbons, four hydrogens, and one oxygen, we add up the masses of all atoms: (2 x 12.0110) + (4 x 1.0078) + 15.9994 = 44.0526 amu.
Examples
To find the relative molecular mass from a mass spectrum, identify the most abundant ion and determine its mass-to-charge ratio. Divide this value by the number of electrons or protons lost to obtain the molecular mass. Several examples and practice problems are available to help build proficiency in calculating relative molecular mass.
| Example Problems | Step-by-Step Guide |
|---|---|
| A mass spectrum shows a peak at 56 and another one at 58. What is the relative molecular mass? | 1. Identify the peaks in the mass spectrum 2. Take the difference between the two peaks 3. The difference between the two peaks is equal to the mass of the repeating unit 4. The relative molecular mass is equal to the mass of the repeating unit multiplied by the number of repeating units in the molecule 5. In this case, the difference between the two peaks is 2, which corresponds to the mass of one carbon atom. 6. Therefore, the relative molecular mass is (56 + 58)/2 x (12.01 g/mol) = 684.36 g/mol |
| A mass spectrum shows a peak at 77 and another one at 79. What is the relative molecular mass? | 1. Identify the peaks in the mass spectrum 2. Take the difference between the two peaks 3. The difference between the two peaks is equal to the mass of the repeating unit 4. The relative molecular mass is equal to the mass of the repeating unit multiplied by the number of repeating units in the molecule 5. In this case, the difference between the two peaks is 2, which corresponds to the mass of one carbon atom and two hydrogen atoms. 6. Therefore, the relative molecular mass is (77 + 79)/2 x (12.01 g/mol + 2.02 g/mol) = 177.35 g/mol |
Common Mistakes To Avoid
When determining the relative molecular mass from a mass spectrum, it is important to avoid common mistakes that can result in incorrect calculations. One common mistake is overlooking isotopes. Isotopes are atoms of the same element with different numbers of neutrons, resulting in a different atomic mass. It is important to account for these isotopes in the calculations. Another mistake is locating the wrong peak as the parent peak. This can lead to incorrect mass values for the compound. Finally, interpreting spectral data incorrectly can also result in erroneous relative molecular mass values. It is important to carefully analyze the spectrum and correctly identify the molecular ion peak and other relevant peaks to accurately calculate the relative molecular mass.
Applications Of Relative Molecular Mass Calculation
| Applications of Relative Molecular Mass Calculation |
|---|
|
Knowing the relative molecular mass of a compound is crucial in medical research, particularly in the development of new drugs. By using mass spectrometry to determine the molecular mass, researchers gain insight into the structure and properties of molecules that are potential drug targets. Additionally, determining the relative molecular mass is important in analyzing chemical compounds and reactions, such as identifying the degradation products of a drug. With this information, chemists can develop more effective and efficient processes to synthesize desired products. Moreover, relative molecular mass calculation is also useful in drug development as it helps ensure the consistency and purity of the product. |
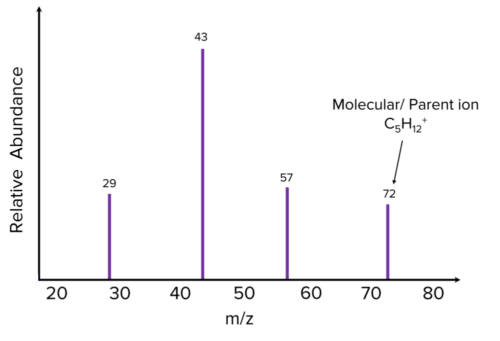
Credit: mmerevise.co.uk
Conclusion
In essence, finding the relative molecular mass from mass spectrum is a fundamental skill that every chemistry student must master. Above all, it is essential to have a basic understanding of mass spectrometry, and its different techniques used for analyzing molecular compounds.
By following the simple steps outlined in this guide, anyone can easily calculate the relative molecular mass from mass spectrum data. Remember, Practice makes perfect, so it’s important to practice this skill regularly to become proficient.
